|
Singapore-MIT Alliance for Research & Technology |
BioSystems and Micromechanics (BioSyM) Inter-Disciplinary Research Group |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
BioSyM Highlights |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
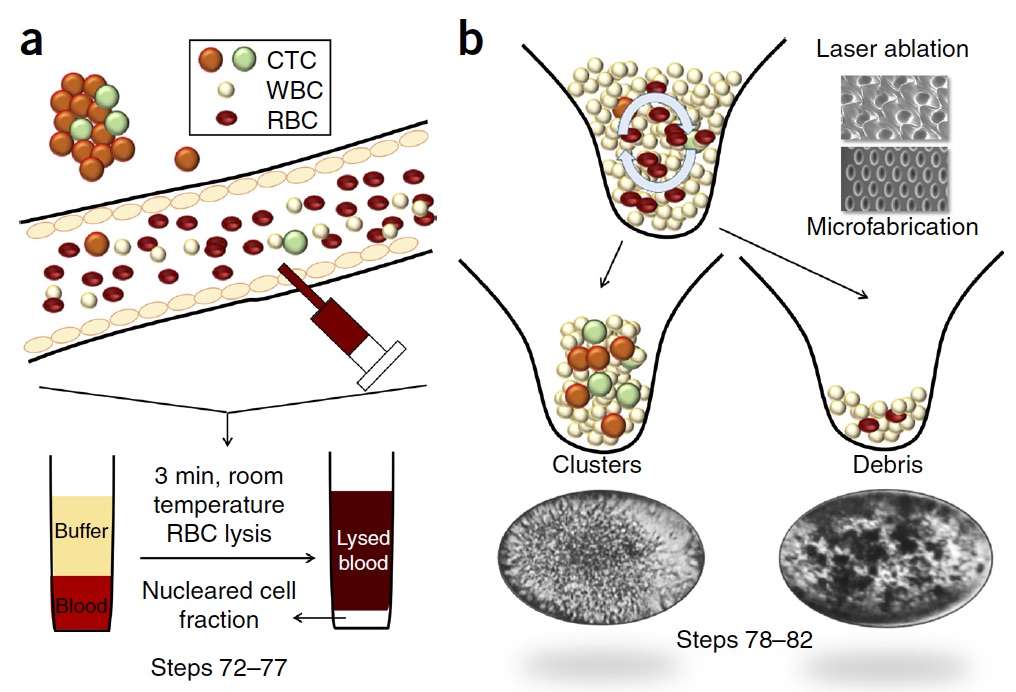
Mechanistic action of weak acid drugs on Biofilms (Scientific Reports, 7; 4783 (2017)): BioSyM and SCELSE (NTU) researchers have studied the efficacy of weak organic acid drug on the eradication of biofilms formed by the mucoid strain of Pseudomonas aeruginosa and investigated the commonality of this drug with that of acetic acid. It is shown that the weak acid can penetrate the biofilm matrix and eventually kill 100% of the bacteria embedded in the biofilm.
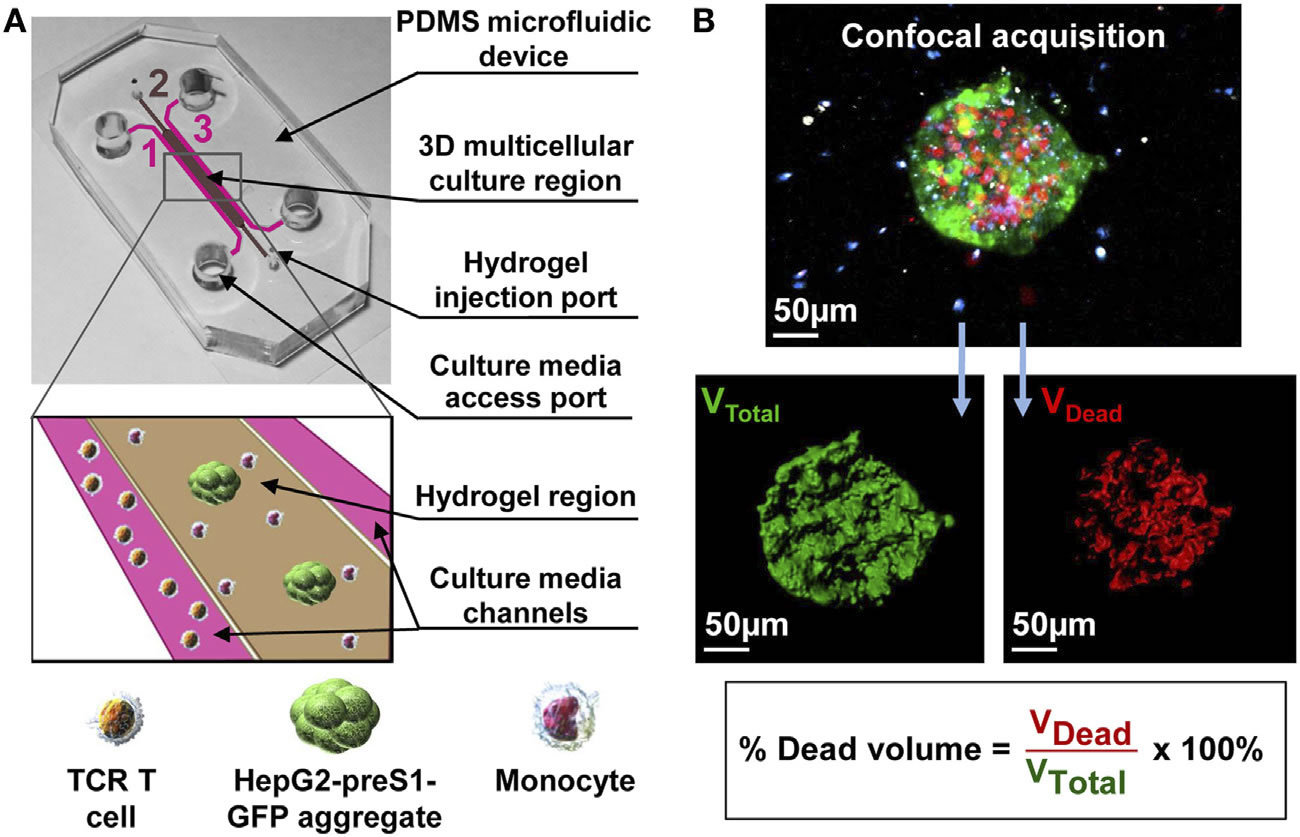
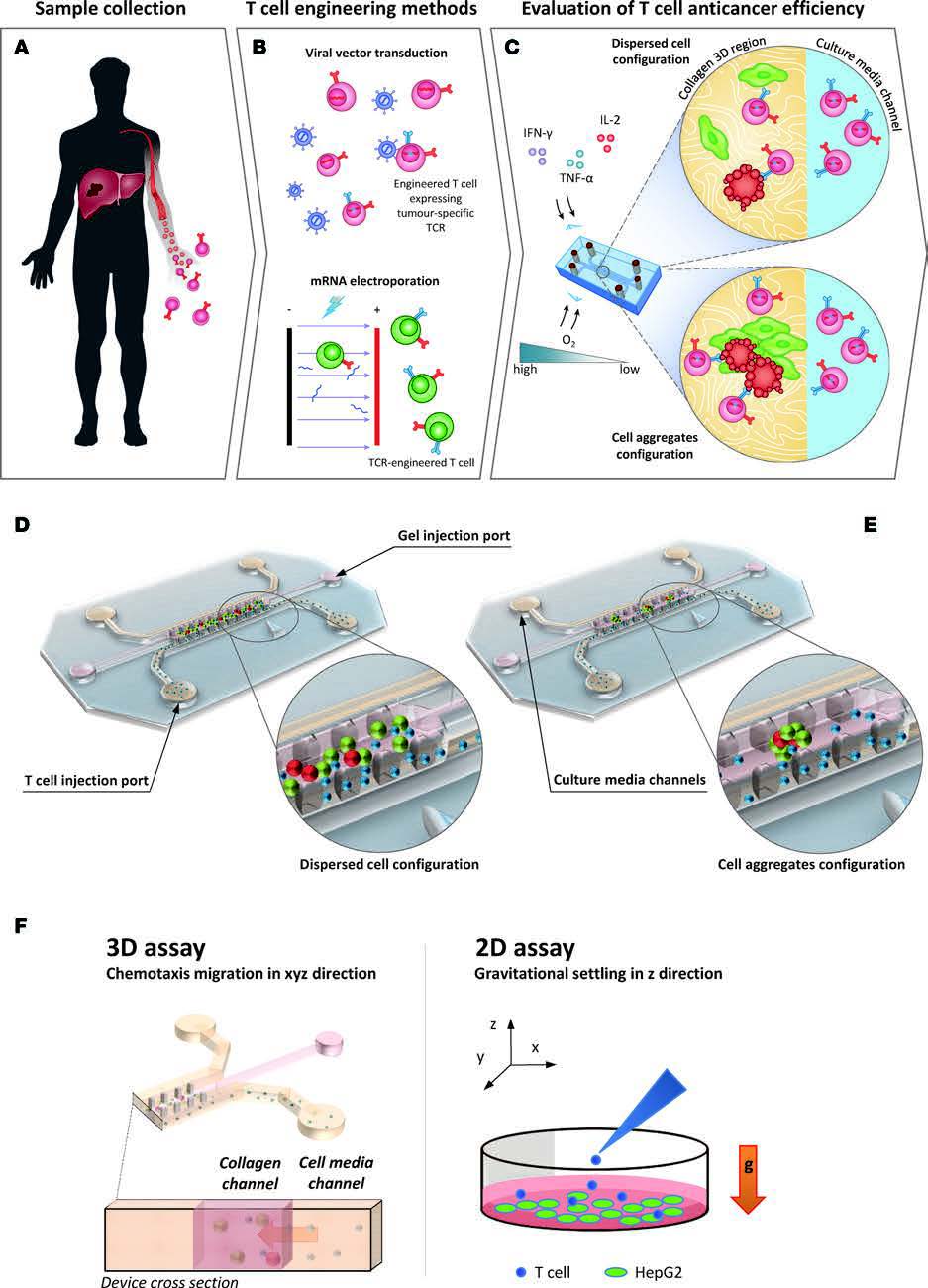
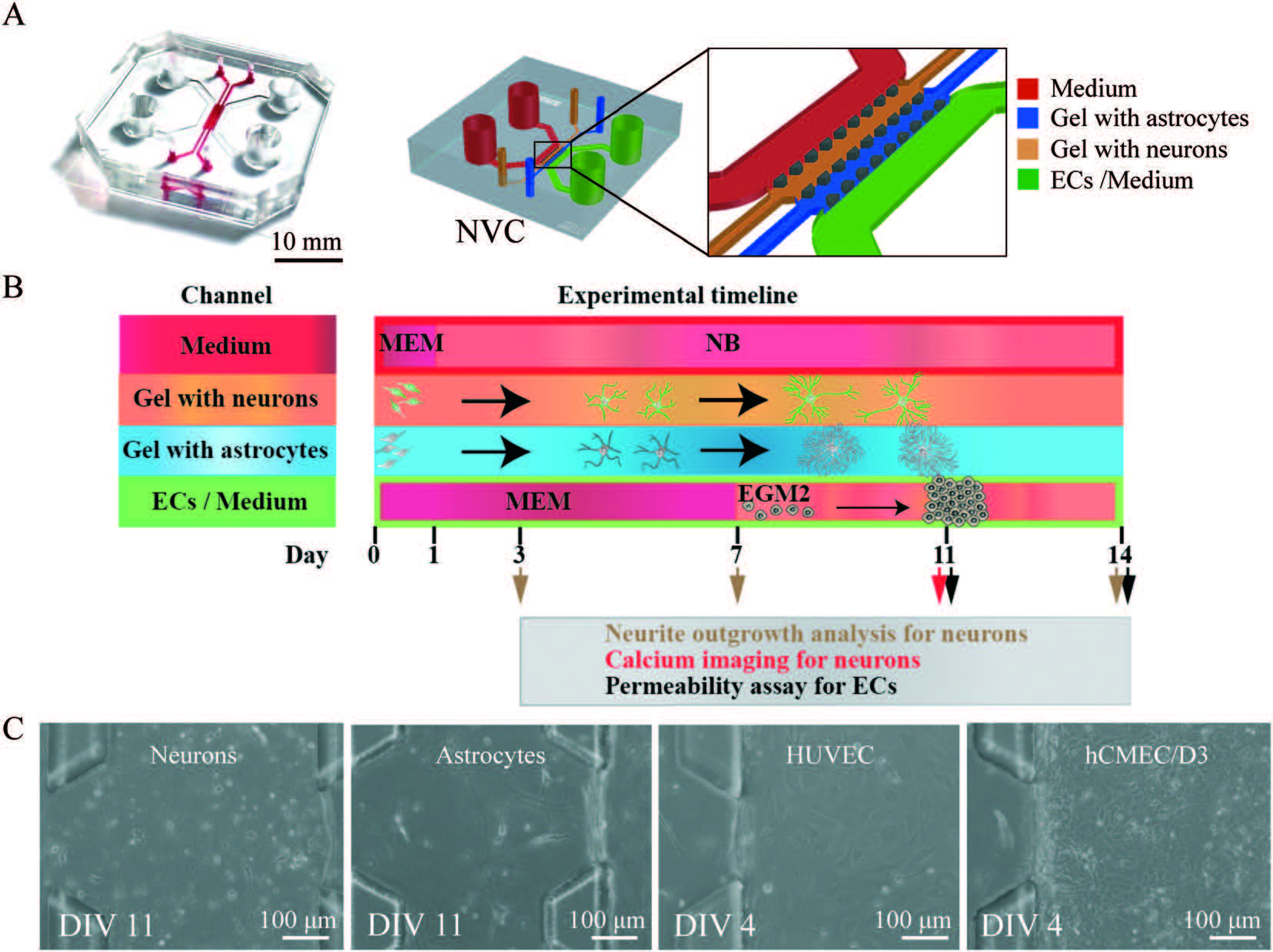
(A) Schematic of a flow cell. (B) Biofilms are grown in flow cells with a glass base and a PDMS top with a continuous supply of 10% Luria-Bertani broth (LB). The drug is injected into the flow cell through the PDMS Microcolonies grow on the glass base to a size of approximately 200 μm after a period of 2 days. A 3D microfluidic model for preclinical evaluation of TCR-engineered T Cells against solid tumors:
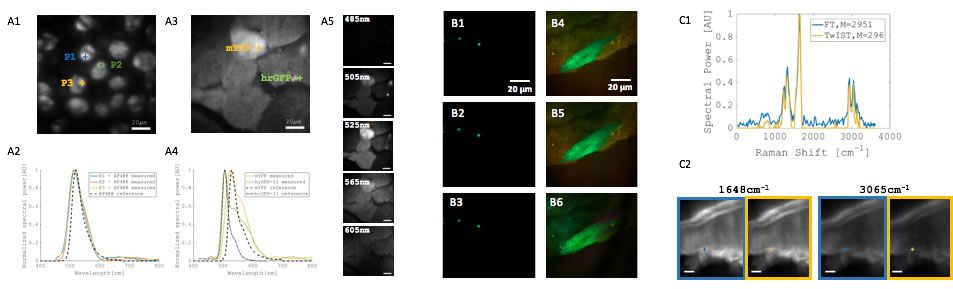
BioSyM researchers at SMART, NUS & MIT have developed a novel interferometer for wide-field imaging using Fourier transform (FT) spectroscopy, which enables many biomedical applications requiring hyperspectral analysis through demonstration on fluorescent beads, cell and tissue specimen.A compressive data acquisition scheme is demonstrated for multiplexed imaging with many fluorescent species. In addition, a wide-field spectral Raman imaging is demonstrated with the new FT interferometer. (Dushan et al "Near-common-path interferometer for imaging Fourier-transform spectroscopy in wide-field microscopy, Optica, 4 (5), May 2017)
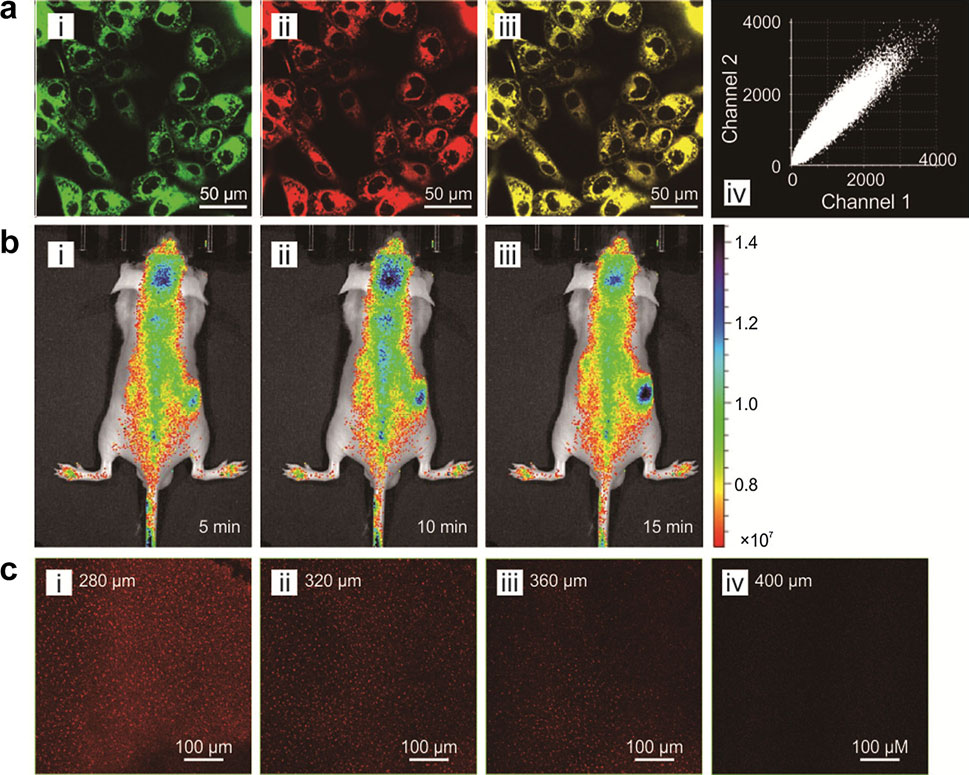
Fluorophores with near-infrared (NIR) emissions play a crucial role in numerous bioimaging and biosensing applications. These NIR fluorophores afford highly attractive optical properties, such as deep penetration depths, good signal-to-noise ratios, and minimal tissue damages. BioSyM researchers Dr. Liu Xiaogang and Professor Matthew Lang with their collaborators have rationally developed a new class of near-infrared fluorophores with bright one-photon and two-photon emissions at ~740 nm, large Stokes shifts (~80 nm), significant two-photon action absorption cross-section (~185 GM at 820 nm), excellent water solubility, outstanding photostability and low toxicity. They also demonstrated their biological applications in mitochondrial labelling, deep tissue imaging and H2S detection in live cells and mice. Their paper is now published online in “Chemistry – an European Journal” and has been selected as a “Hot Paper” by the Editor. "Hot Papers are chosen by the Editors for their importance in a rapidly evolving field of high current interest”.
BioPhysical Society TV 2017 has featured a video on BioSyM released during their recent event "BioPhysical Society 61st Annual Meeting (https://www.biophysics.org/2017meeting/Home/tabid/6672/Default.aspx), held from Feb 11-15, 2017, New Orleans, Louisiana, USA.
STRAITS TIMES reports BioSyM Research: "Singapore-made device to aid in developing cancer therapy"
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
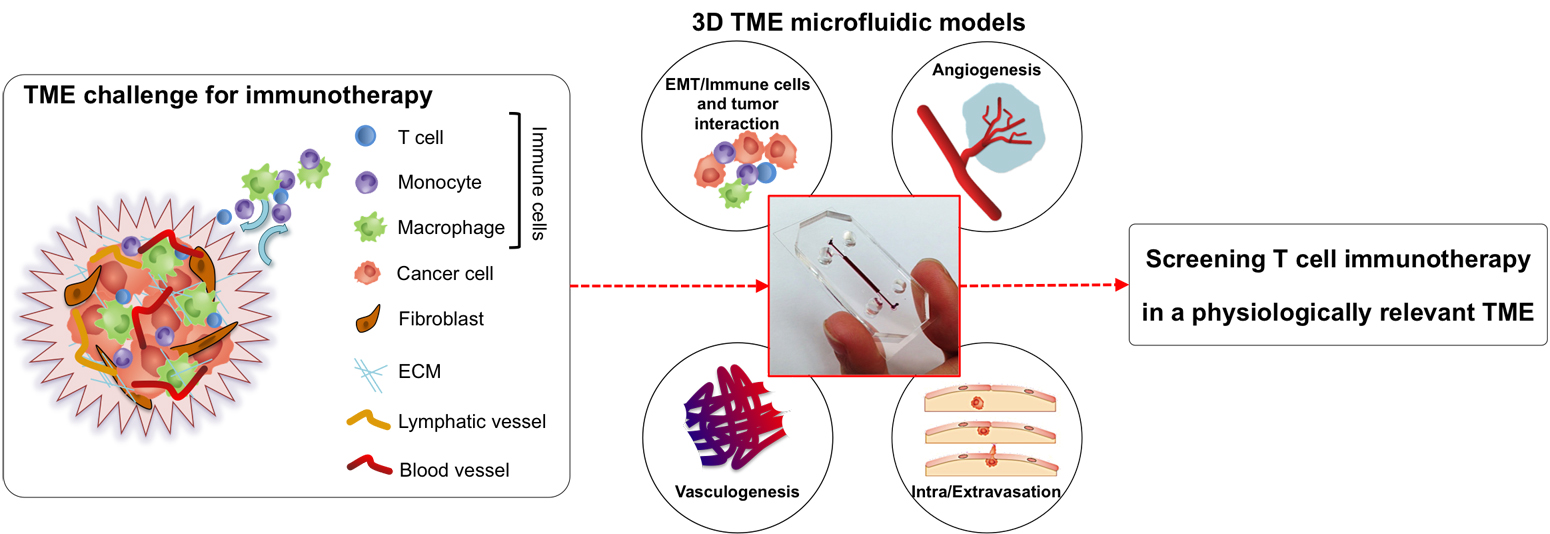 |
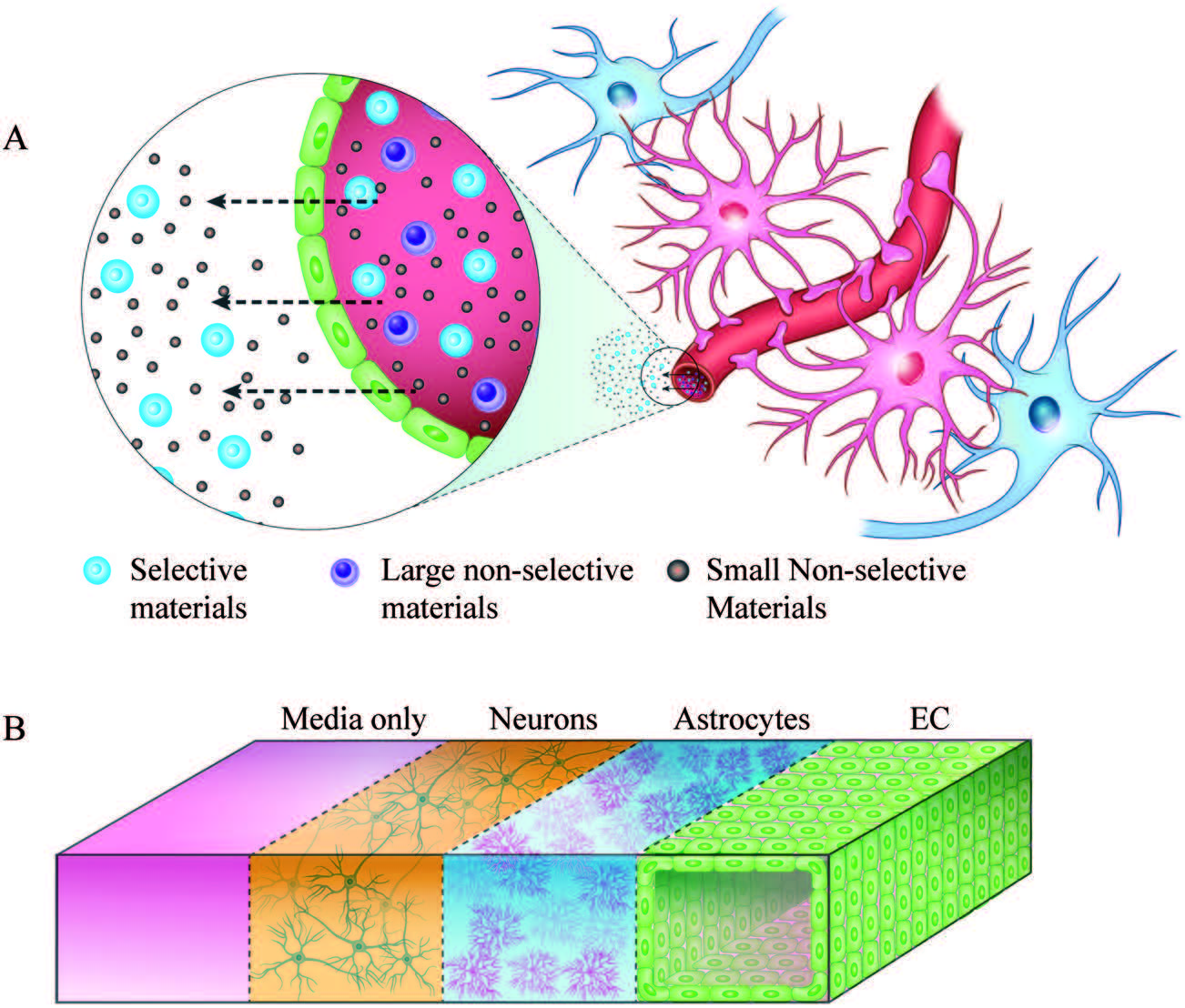
| Schematic representation of the tumour microenvironment (TME) and its main constituents that represent a challenge for T cell immunotherapy (left). 3D TME microfluidic models used to study cancer metastasis and tumor-immune system interaction mechanisms (center) represent a preclinical screening platform for T cell immunotherapies. |
BioSyM researcher awarded Open Fund - Young Individual Research Grant (YIRG)
 |
BioSyM Post-Doctoral researcher Dr.Khoo Bee Luan has been awarded the NMRC’s Open Fund - Young Individual Research Grant for advancing her research work on “Microfluidic Assay for Routine Evaluation of Anti-Cancer Drug Therapy on Clinical Human Circulating Tumor Cells”. The outcome of this work will be impactful not only in terms of elucidating the mechanism of cancer metastasis, but also finding many new clinical counter-measures to combat the significant challenge of metastatic cancer in clinics |
 |
BioSyM & MBI researchers have demonstrated an efficient approach to evaluate response to cancer drug using patient-derived circulating tumor cell (CTC) cultures obtained from liquid biopsy. This new and robust liquid biopsy technique can potentially evaluate patient prognosis with CTC clusters during treatment and provide a noninvasive and inexpensive assessment that can guide drug discovery development or therapeutic choices for personalized treatment. "Liquid biopsy and therapeutic response: Circulating tumor cell cultures for evaluation of anticancer treatment", Science Advances, 2 : e1600274 (2016) |
SMART-BioSyM advances cancer therapy – Tumour Treating Fields – with a novel microfluidic device
 |
- Tumour Treating Fields (TTFields) are low intensity, alternating electric fields that disrupt cell division through physical interactions with key molecules during mitosis. This non-invasive treatment targets solid tumours and has been FDA-approved for Glioblastoma (brain cancer).
Details published in Engineering a 3D microfluidic culture platform for tumor-treating field application", Scientific Reports |
| (Top, L to R): PDMS microfluidic device with embedded electrodes; Lung cancer aggregates in the 3D hydrogel within device, in co-culture with non-cancerous cells; Injecting lung cancer cells into device (Bottom pix): Researchers Andrea and Giulia showing images of stimulated & dispersed non-stimulated cancer aggregates | |
Guided by quantum chemical calculations, BioSyM researchers, Liu Xiaogang (SMART Scholar) and Matthew Lang (PI), in collaboration with scientists from Chinese Academy of Sciences, have demonstrated a simple chemical substittution that greatly enhances fluorophore performance. They also revealed two major mechanisms that compromise the brightness and photostability of fluorophores. Such knowledge is a critical step towards developing high-performance fluorophores for advanced fluorescence imaging. Their results have been published in Journal of the American Chemical Society, one of the most prestigious chemistry journals.
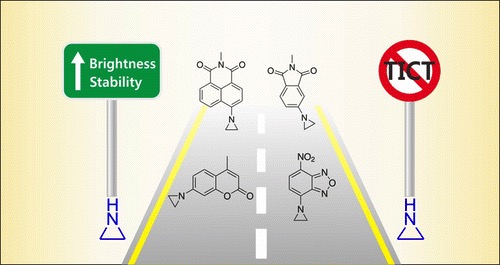 |
| X. Liu*, Q. Qiao, W. Tian, W. Liu, J. Chen, M. J. Lang, Z. Xu*, “Aziridinyl fluorophores demonstrate bright fluorescence and superior photostability through effectively inhibiting twisted intramolecular charge transfer”, Journal of American Chemical Society, 2016, DOI: 10.1021/jacs.6b03924. |
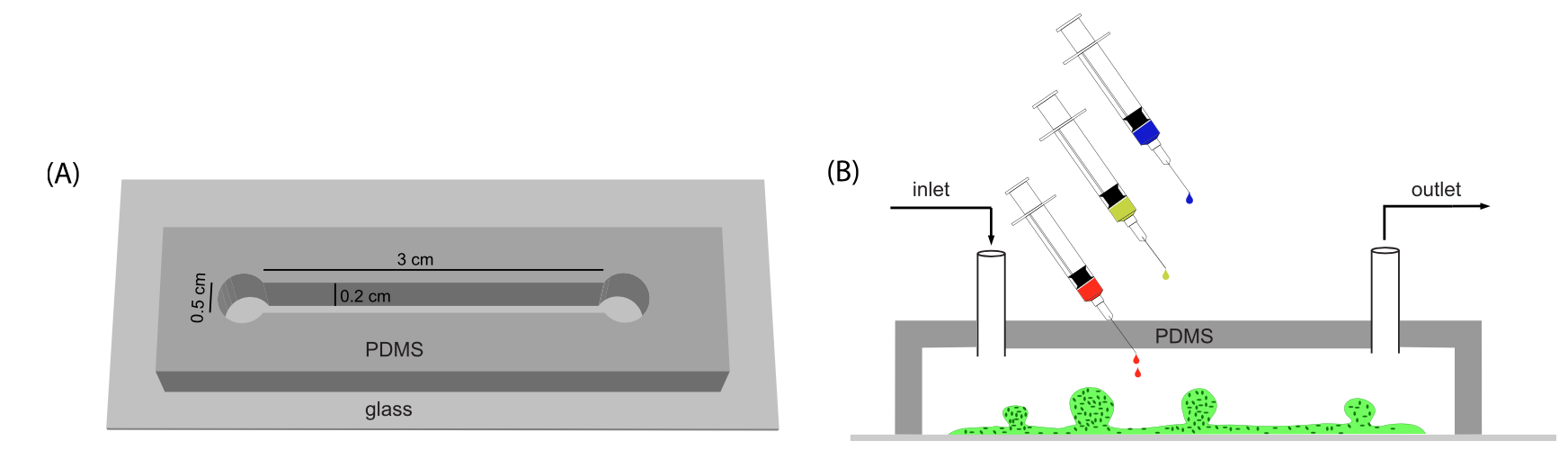
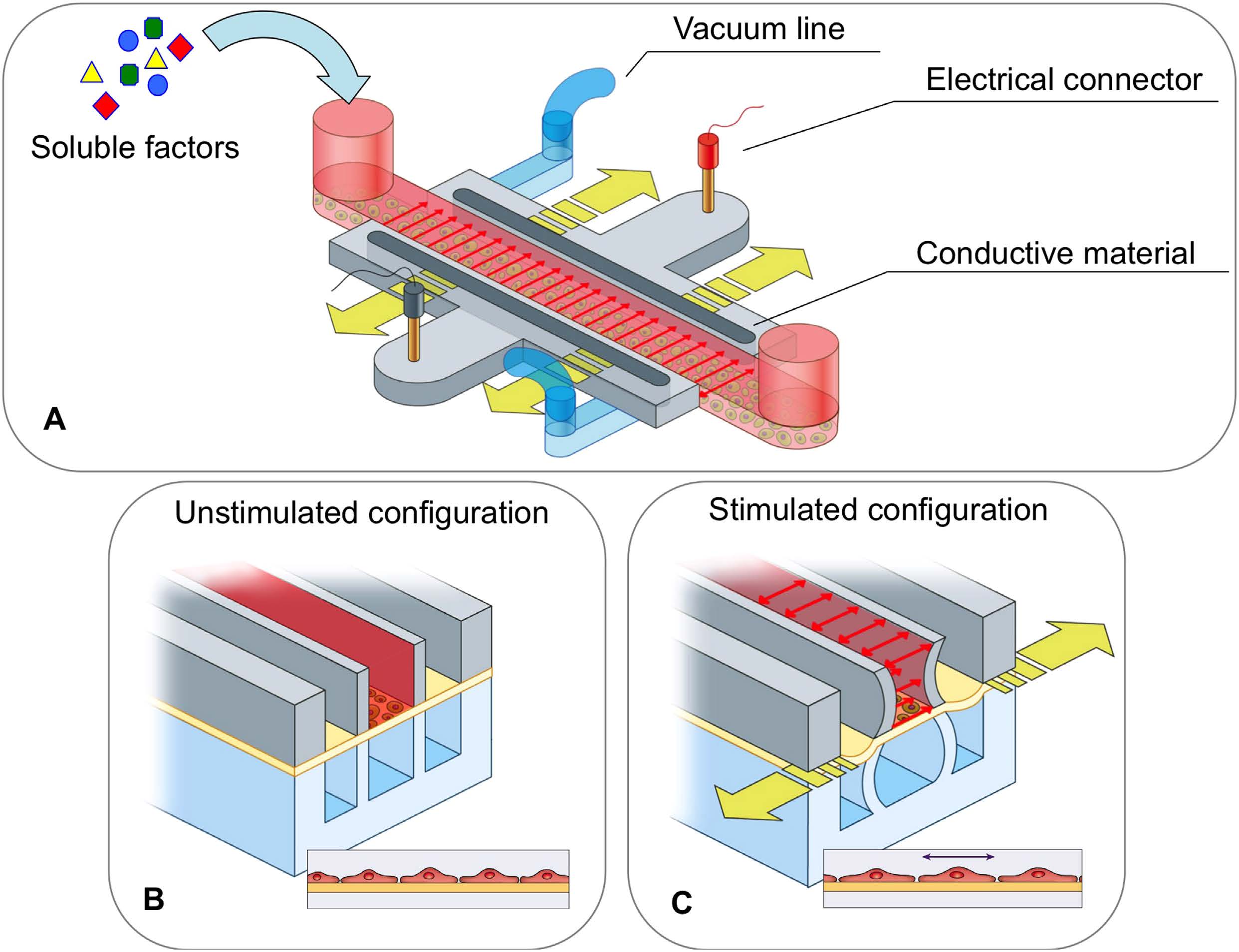
BioSyM researchers and collaborators have demonstrated a novel microfluidic device with embedded electrodes that enables the application of an alternating electric field therapy to cancer cells in a 3D extracellular matrix. The metastatic potential of the cancer cells and the proleferation rate were reduced after electric field treatment. These results form the basis for the potential use of both Chemotherapy and ElectricFieldTherapy in frontline cancer treatment regimens. {"Engineering a 3D microfluidic culture platform for tumor-treating field application", Scientific Reports}
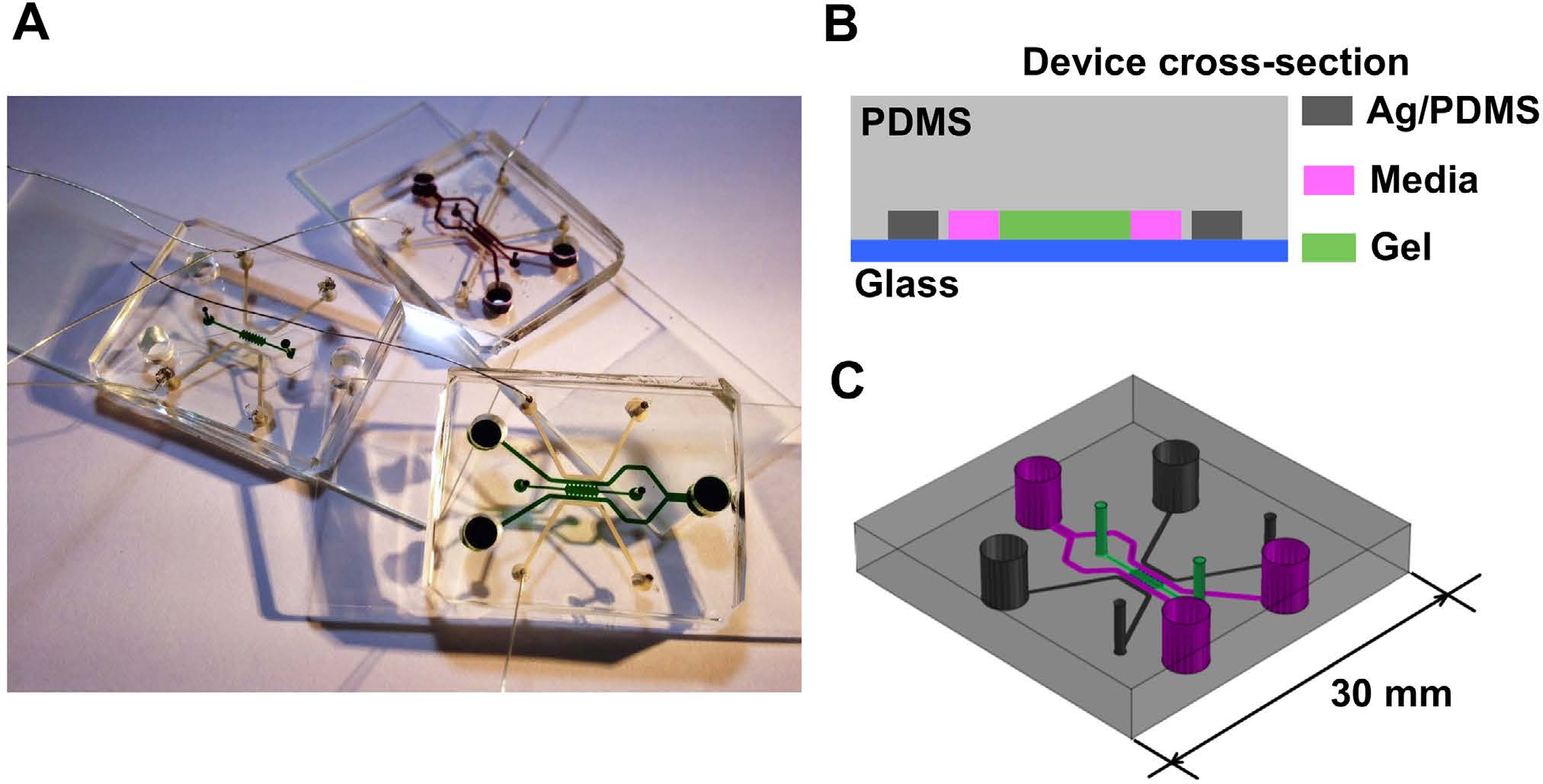 |
 |
Microfluidic device for Electric Field application. (A) Photo of three devices filled with food color dyes. Device cross section (B) and 3D CAD overview (C) with the conductive mixture (Ag/PDMS) in dark grey, cellculture media in pink, and 3D hydrogel region in green. |
Equivalent electric circuit model of the microfluidic device |
 |
| Schematic representations of carcinoma aggregate dispersion at 0h and 36h in contact and separate condition into the microfluidic device. First column shows the macrophage subtype and the microfluidic device (media channel in pink, media channel with HUVECs in green, 3D collagen matrix for carcinoma aggregate and macrophage interactions). Second and third columns show the schemes for the contact or the separated condition at 0h and 36h (A549 aggregates in red; M0, M1, M2b and M2c in green; M2a in blue) |
BioSyM and MBI researchers have evaluated the Single Cell Analysis (SCA) technique to address heterogenity issues associated with cancer cells. The complete review is now published in "International Journal of Cancer"
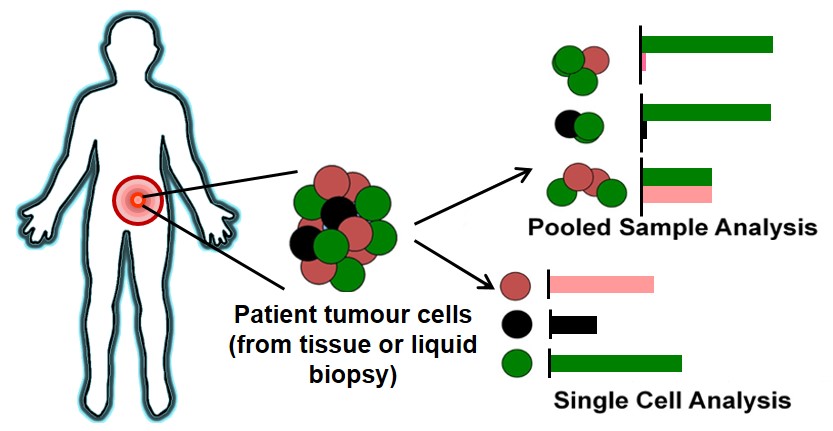 |
| The illustration covers the process of tumor biopsy from a patient, to downstream characterization using pooled cell analysis or single cell techniques. The bar charts demonstrates the frequency of gene/protein expression detected; pooled sample analysis incorporates several tumor cell subpopulations, which may mask signals or lead to inaccurate display of relative expression levels, while single cell analysis allows breakdown to reveal individual heterogeneity for a more accurate assessment. |
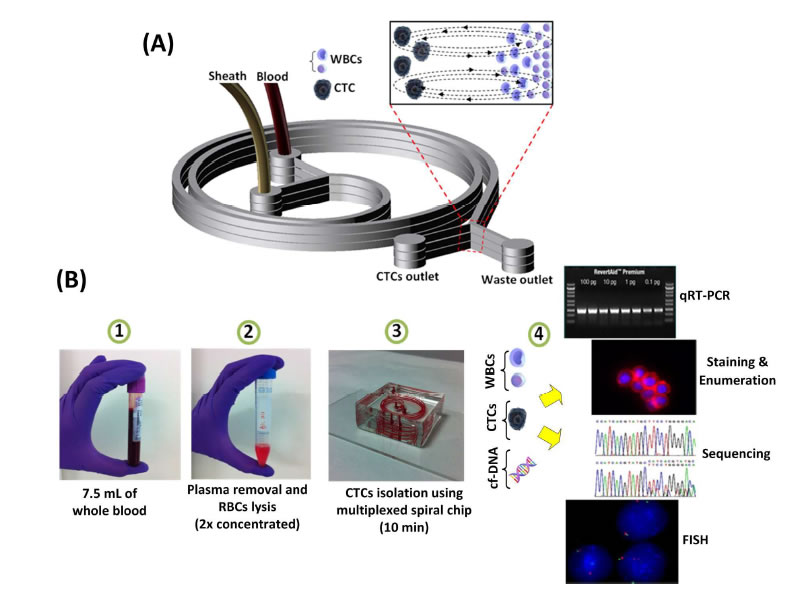
BioSyM research published in Nature Protocols, Jan 2016 issue: Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics, Majid Ebrahimi, et al.,
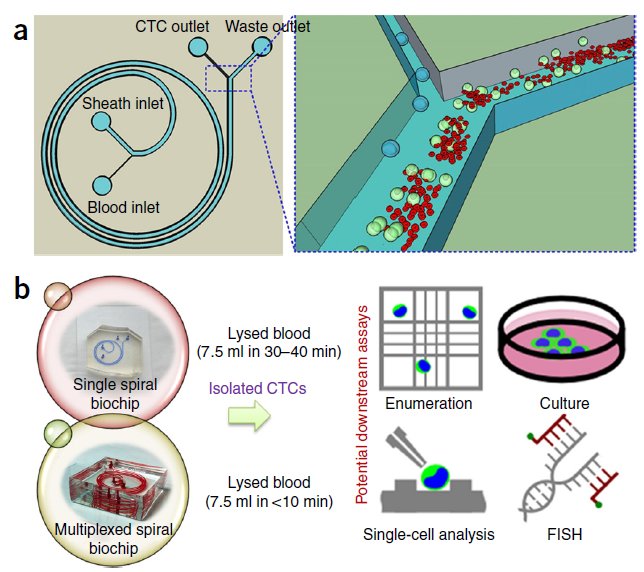 |
Overview of CTC isolation using spiral microfluidics. (a) Schematic of CTC enrichment by a spiral channel. CTCs are focused near the inner wall, whereas WBCs, RBCs and platelets are focused closer to the outer wall at the outlet because of the combined effects of inertial lift force and Dean drag force. (b) Left, optical image of single and multiplexed spiral biochips for high-throughput CTC isolation from lysed blood. Right, schematic illustrating the possible downstream techniques for functional characterization of CTCs isolated using a spiral biochip. |
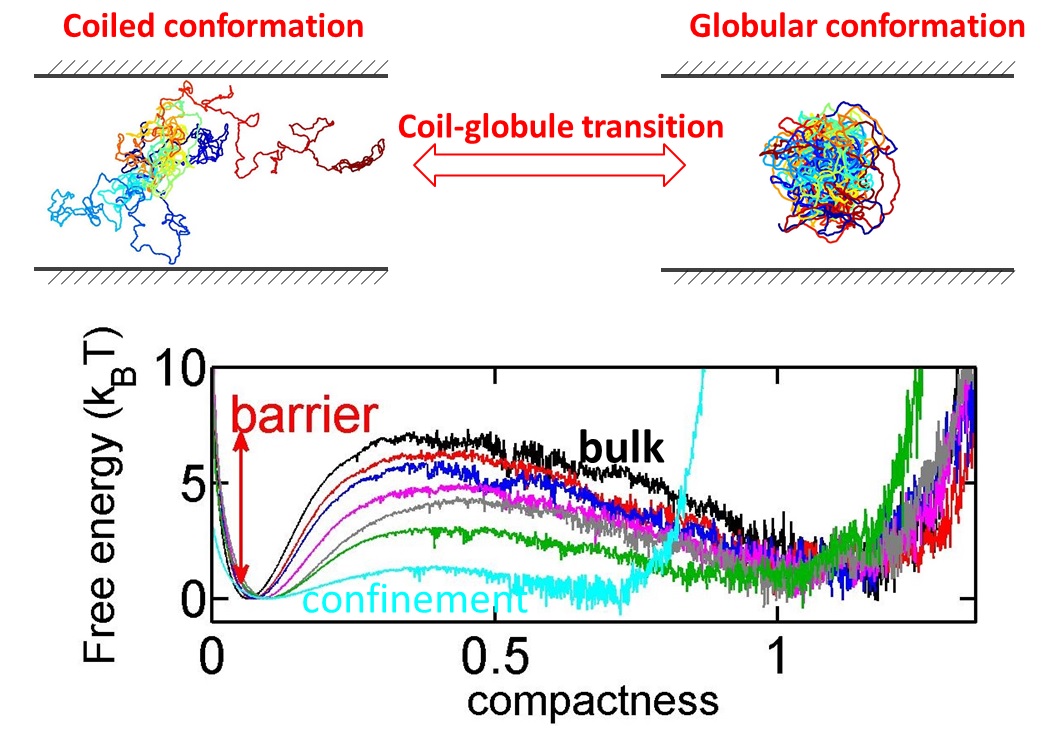
Protein folding and DNA condensation are two important instances that a biopolymer undergoes a conformation change from a coiled state to a globular state. Such coil-globule transition often occurs in confined space due to cell membranes.BioSyM researchers have found that confinement makes the transition easier and faster, because the coiled conformation is disfavored in confined space. The details are published in Scientific Reports(Nature Publications)
BioSyM Alumni Dr.Majid Ebrahimi Warkiani has been chosen as one of the 10 Honourees of MIT TR35 Innovators Under 35 Asia. Majid was one of the major contributors to BioSyM's research on Microfluidic Cell sorting and its application to sorting Circulating Tumor Cells (CTCs).

http://www.emtechasia.com/index.php/innovators-under-35/tr35-honourees
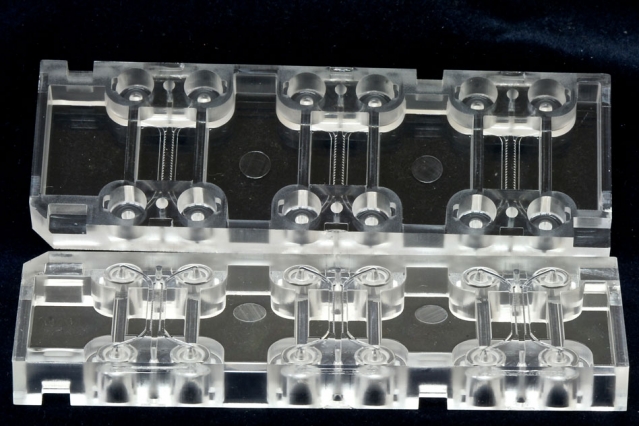 |
SMART-BioSyM start-up, AIM Biotech's microfluidics device (shown here) has an array of culturing sections, each with three chambers: a middle chamber for hydrogel and any cell type, and two side channels for culturing additional cell types. |
Spinout’s microfluidics device better models how cancer and other cells interact in the body: https://www.aimbiotech.com/ |
|
BioSyM - A*STAR - NUS researchers develop microfluidic "in vitro" platform to study Tumor Associated Macrophages (TAMs)
Researchers from SMART-BioSyM and A*STAR Institutes SIgN and IMCB have developed a novel microfluidic based in vitro tumor microenvironment. This platform was utilized to study the role of individual subtypes of macrophages (M0, M1, M2a, M2b and M2c) in human lung adenocarcinoma (A549) aggregate dispersion, as a representation of epithelial- mesenchymal transition (EMT). The findings may help in the development of immunotherapies based on enhancing the tumor-suppressive properties of TAMs. The results are now published in Oncotarget.
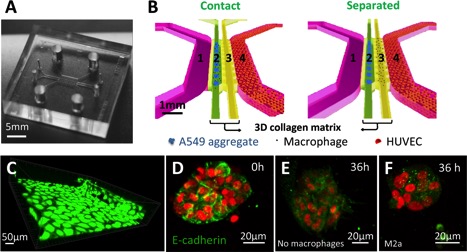 |
|
Membrane-less microfiltration using inertial microfluidics
BioSyM researchers have developed a membrane-less microfiltration system by massively parallelizing inertial microfluidics to achieve a macroscopic volume processing rates (~ 500 mL/min). This platform has the desirable combinations of high throughput, low-cost, and scalability, making it compatible for a myriad of microfiltration applications and industrial purposes. The details are described in Scientific Reports (Nature Publishing) 5, 11018 (2015)
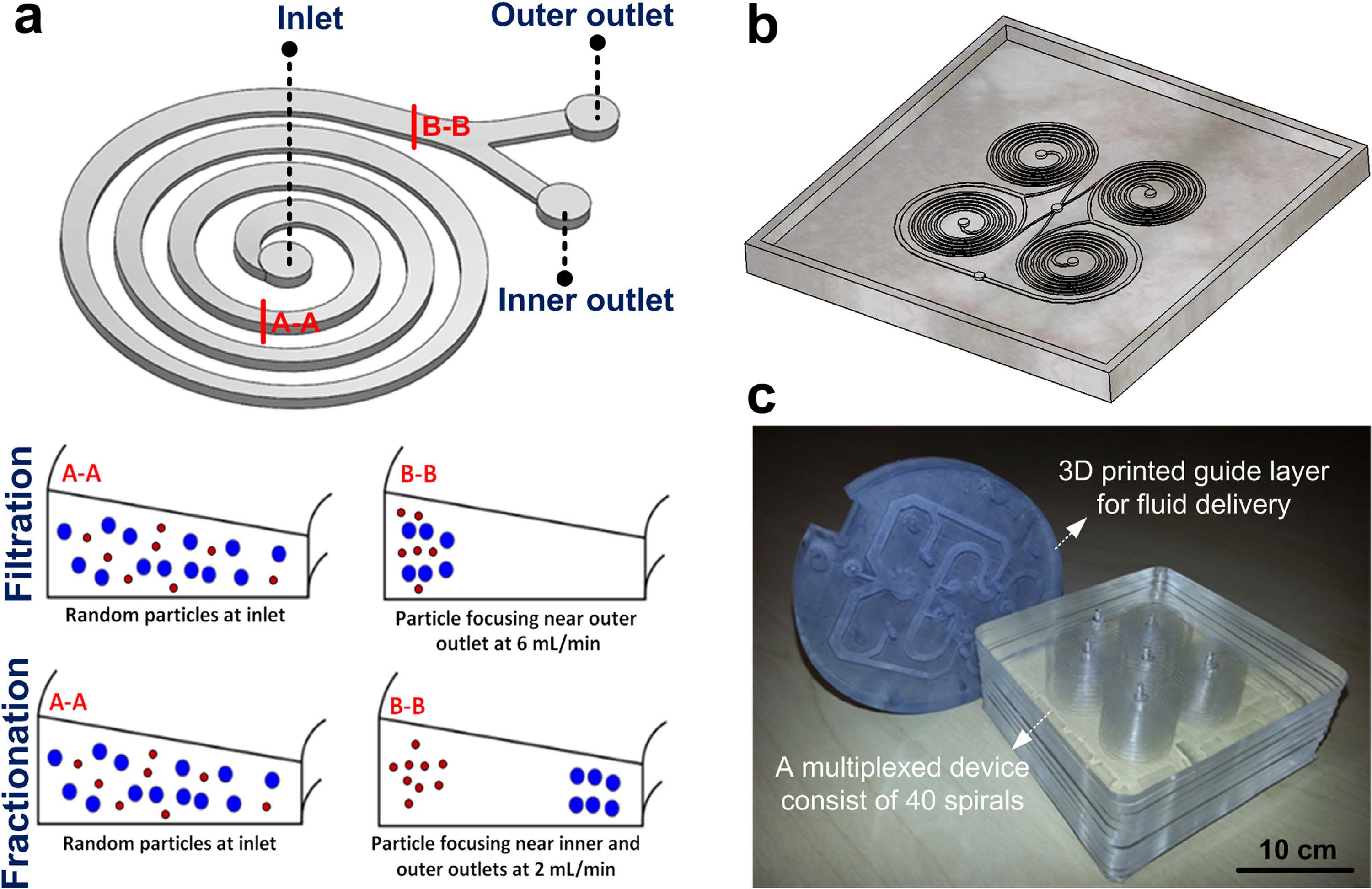 |
|
Controlled electrical, mechanical and biochemical stimulation of Cells on a chip
BioSyM researchers describe in a new publication in Scientific Reports (Nature Publishing) 5, 11800 (2015), the design and fabrication of a microfluidic device capable of simultaneously providing mechanical, electrical, and biochemical stimulation, and subsequently extracting detailed morphological and gene-expression analysis on the cellular response.
Enhancing malaria diagnosis through microfluidic cell enrichment
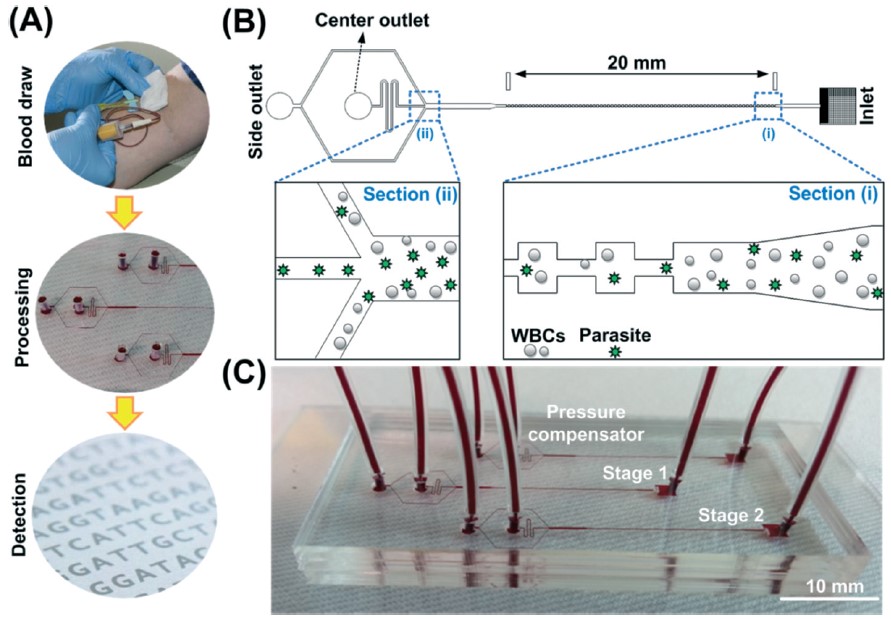
BiosyM researchers have demonstrated an approach to increase the sensitivity and reliability of malaria diagnosis by Magnetic Resonance Relaxometry (MRR) using a microfluidic cell enrichment technique. The details are published in Scientific Reports, 5: 11425 (2015)
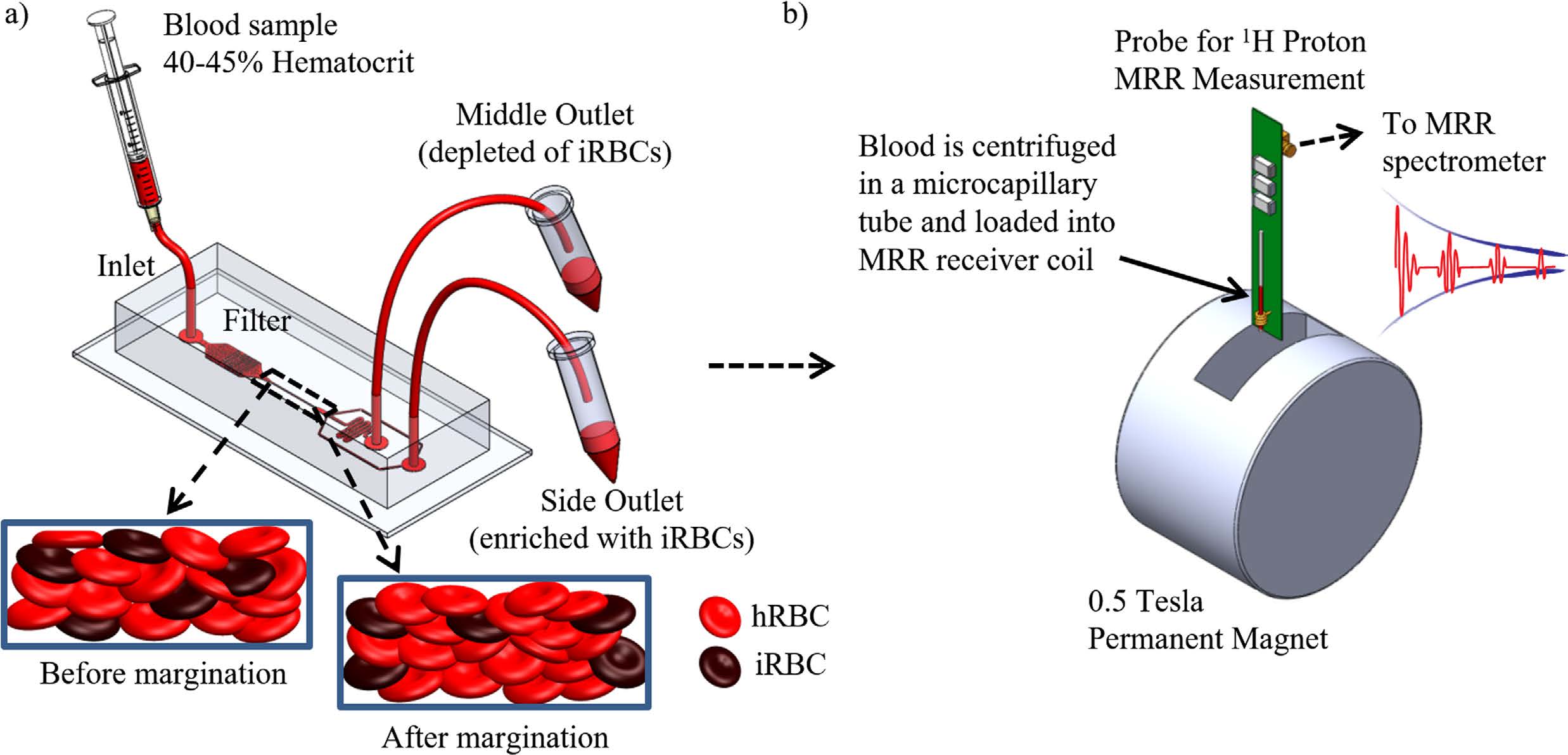 |
|
PREDICTING PATIENT RESPONSE TO CANCER TREATMENTS VIA MONITORING CULTURES OF CIRCULATING TUMOR CELLS
A collaborative study led by scientists from the Mechanobiology Institute (MBI), Singapore MIT Alliance for Research and Technology (SMART) - BioSystems and Micromechanics (BioSyM) and the National University Hospital at the National University of Singapore has led to the development of a novel technique for culturing circulating tumor cells (CTCs). This assay could be used for predicting cancer treatment outcome. Their work is published in Oncotarget on 6 May 2015.
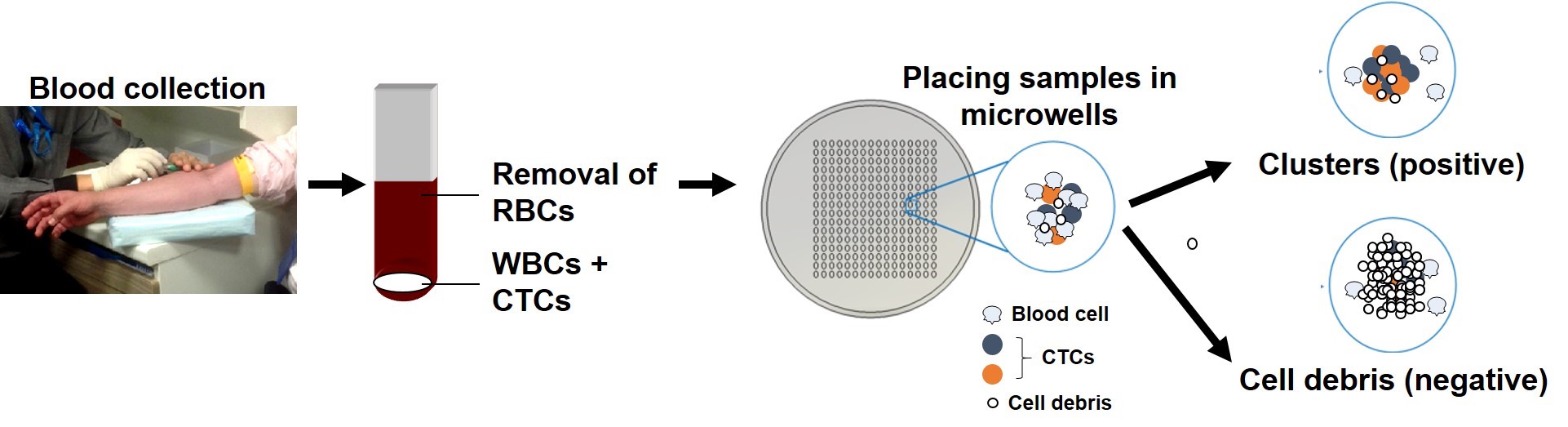
BioSyM's research was displayed at Singapore's 1st Tech Carnival on 25th April 2015 @ Suntech City Tower, as part of SMART's research showcase on healthcare, environment and mobility. The video displayed 4 of BioSyM's projects: Miniaturised Magnetic Resonanance Relaxometry for malaria detection, Bio-Imaging, Microfluics Cell sorter and 3D microfluidics for drug screening
International Media visits SMART BioSyM labs @ CREATE
Biosym hosted a group of International Media for a lab visit on 20th April 2015. BioSyM researcher Dr.Andrea Pavesi and his collaborator from Duke-NUS Dr.Tan Anthony Tanoto described our 3D microfluidic technology and its clinical applications in cancer treatment. Mr.Kuan Chee Mun, CEO of AIM Biotech described his company's path in commercializing BioSyM's technology.

Malaria detection using inertial microfluidics
BioSyM researchers have developed a label-free, shear-modulated inertial microfluidic system to enrich malaria parasites from blood so as to facilitate a more reliable and specific PCR-based malaria detection. The technique is 100X more sensitive than the gold standard conventional microscopy analysis of thick blood smears. The details are published in the journal Lab On A Chip.
Origin of Metastable Knots in Single Flexible Polymer Chains
Long polymer molecules such as DNA can find themselves in knotted conformations. BioSyM researchers develop a new theory for the size distribution of knots on a flexible polymer such as DNA. Knotted DNA molecules present interesting phenomena in the process of sequencing genomes during nanopore translocation. The work is published now in Physical Review Letters, 114, 037801 (2015)
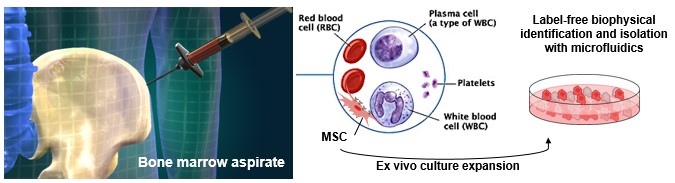
Cell therapy in pre-clinical models of bone marrow injury
BioSyM researchers demonstrate the Mesenchymal Stem Cell (MSC) subpopulation’s increased efficacy as a systematically administered cell therapy in preclinical models of bone marrow injury. Compared to survivability of 0% using current “state of the art” but functionally heterogeneous MSCs, this MSC subpopulation achieved >85% survivability. This study highlights the opportunity for rapid clinical translation of BioSyM's cell sorting approach and of this MSC subpopulation for several emerging applications of MSC regenerative medicine. The details of this work is now published in Stem Cells Translational Medicine.
World’s first novel method for label-free identification of stem cells that will lead to more consistent and efficacious stem cell therapies
Left Pix: (L-R) Associate Professor Jerry Chan, SMART co-Investigator; Dr Zhiyong Poon, SMART Research Scientist and Dr Jacky Lee, former SMART Postdoctoral Associate, with the microfluidics cell sorting device that isolates Mesenchymal Stem Cells (MSCs) for bone and muscle therapeutic applications. This device can reduce the analysis and decision-making process from 1 - 2 months to less than 3 days. Right Pix: Close-up of the microfluidics cell sorting device.The research entitled ‘Multivariate biophysical markers predictive of mesenchymal |
|
A new way to diagnose malaria
SMART-BioSyM team has now come up with a possible alternative. The researchers have devised a way to use magnetic resonance relaxometry (MRR), a close cousin of magnetic resonance imaging (MRI), to detect a parasitic waste product in the blood of infected patients. The paper describing the technique in the Aug. 31 issue of Nature Medicine.
http://newsoffice.mit.edu/2014/new-method-diagnose-malaria-0831
SMART- BioSyM Press Release

http://smart.mit.edu/news-a-events/press-room/article/49-scientists-in-singapore-develop-groundbreaking-technique-for-early-and-rapid-malaria-diagnosis.html


Worldwide News coverage on our Malaria diagnosis technique
http://www.nature.com/news/quick-test-for-malaria-shows-promise-1.15796
http://www.natureasia.com/en/research/highlight/9416
http://www.bangkokpost.com/news/world/429845/new-test-fast-tracks-diagnosis-for-malaria
http://www.straitstimes.com/news/asia/south-east-asia/story/new-test-fast-tracks-diagnosis-malaria-20140901
http://medicalxpress.com/news/2014-08-malaria-magnetic-fields.html
http://www.eurasiareview.com/01092014-new-way-diagnose-malaria/
http://www.bioportfolio.com/news/article/2048657/Micromagnetic-resonance-relaxometry-for-rapid-label-free-malaria-diagnosis.html
http://www.thephuketnews.com/new-test-fast-tracks-diagnosis-for-malaria-48493.php
http://www.newsmobile.in/articles/2014/09/01/new-test-fast-tracks-diagnosis-malaria
http://www.dailynewsen.com/science/magnetic-detection-of-malaria-shows-promise-h2590320.html
http://www.sciencedaily.com/releases/2014/08/140831150339.htm
http://www.ibtimes.com/scientists-invent-inexpensive-quick-accurate-malaria-test-1674922http://www.deutschlandfunk.de/medizin-ein-schnelltest-fuer-malaria.676.de.html?dram:article_id=296226
In Vivo, Label-free Imaging of liver surface using multi-photon microscopy
BioSyM researchers and collaborators from A*STAR-IBN and NUS have developed a label-free, three-dimensional quantitative and sensitive method to visualize various structural features of liver surface in living rat using multi-photon microscopy (MPM). The work is now published in Applied Physics Letters.
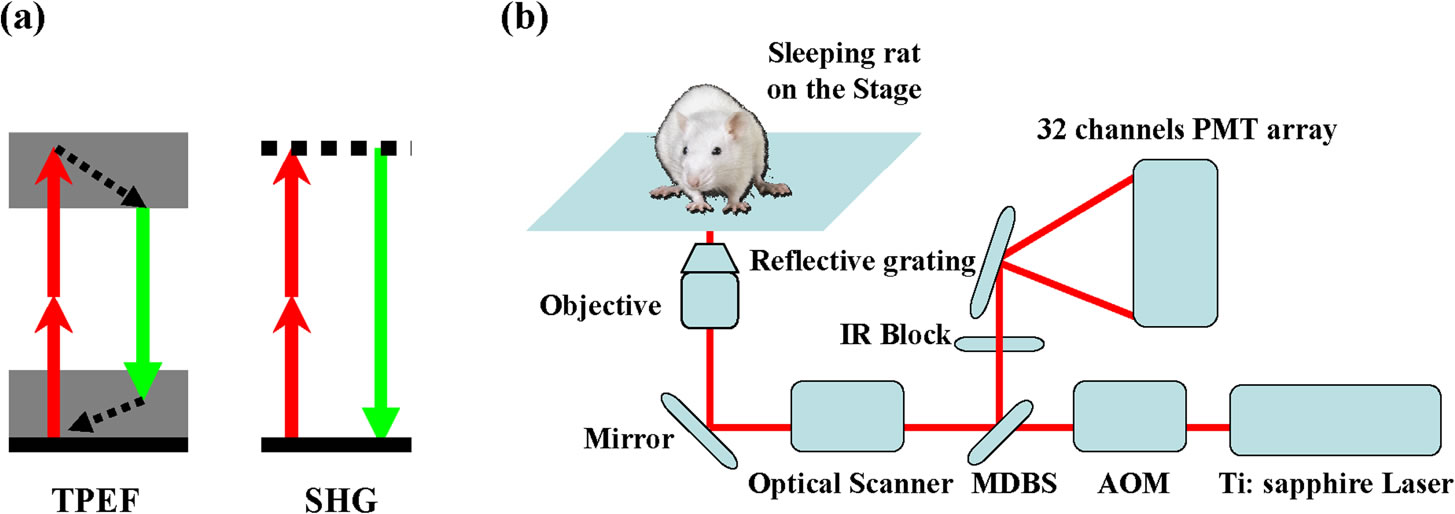
(a) Principle of the TPEF / SHG (two-photon excited fluorescence / Second-harmonic generation) (b) Schematic of the experimental setup.
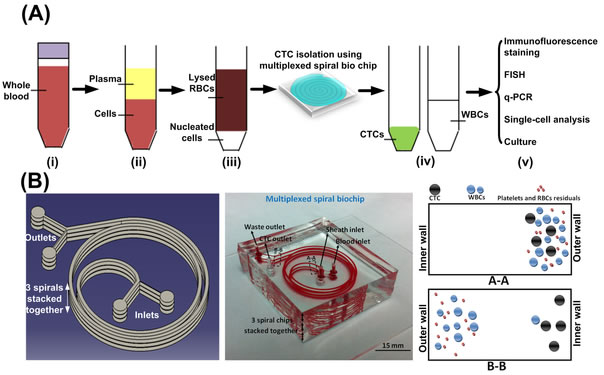
Clinical Validation of an Ultra High-Throughput Spiral Microfluidics for the Detection and Enrichment of Viable Circulating Tumor Cells
Multi-organizational collaborative study among researchers from SMART-BioSyM, NUS, MBI (Mechanobiology Institute), NCCS (National Cancer Centre, Singapore), Clearbridge Biomedics, Sequenom Inc. USA, SGH (Singapore General Hospital), NUH (National University Hospital) has clinically vaildated our multiplexed microfluidic chip for the ultra high-throughput, low-cost and label-free enrichment of CTCs. Retrieved cells were unlabeled and viable, enabling potential propagation and real-time downstream analysis using next generation sequencing (NGS) or proteomic analysis (PLoS One, PloS One, 9(7), e994092014. (2014))
 |
Congratulations !!
 |
|
Dr. Jacky Lee, a NUS / BioSyM Graduate Student was awarded the Chua Toh Hua Memorial Gold Medal 2013/2014 for the best PhD thesis in the life sciences. Jacky Lee carried out his dissertation work @ BioSyM labs under the supervision of Prof.C.T.Lim (NUS), Prof.Jongyoon Han (MIT/SMART) and Prof.Krystyn Van Vliet (MIT/SMART). He is currently with Ministry of Health, Singapore. |
Mr. Andy Tay, an undergraduate student under the supervision of BioSyM Research Fellow Dr Majid EW, has won this year’s Students’ Design Gold Award at the Biomedical Engineering Society, Singapore – 8th Scientific Meeting 2014. Andy won the Gold Medal for the Students’ Design Competition with his project carried out in BioSyM on "Microfluidic Device for Malaria Diagnosis for resource-scarce regions". With the award, Andy will attend the International Federation of Medical and Biological Engineering (IFMBE) Sponsored Asian-Pacific Medical Device Design Competition 2014 during the 9th Asian-Pacific Conference on Medical and Biological Engineering (APCMBE 2014) at National Cheng Kung University, Tainan, Taiwan, from October 9 to 12, 2014, representing Singapore Biomedical Engineering Society with the same project. |
NUH - SMART BioSyM Collaboration
A collaboration between NUH and SMART has developed a percentile chart using the labile glycated haemoglobin A1c (a precursor of HbA1c) to HbA1c ratio to help identify factors that may give rise to potentially spurious HbA1c results. Glycated haemoglobin (HbA1c) is the recommended biomarker for monitoring the glucose control of patients with diabetes. However, the measurement of HbA1c can be adversely affected by several confounding factors. The details of the work has been published in the Journal of Clinical Pathology: doi: 10.1136/jclinpath-2014-202346.
Prof.Harry Asada addressing the participants of the BioSyM Short Course on "Biomedical Image Processing with MATLAB" (9th June 2014) @ CREATE
- BioSyM's work in the Journal "Analyst" gets the cover page
-
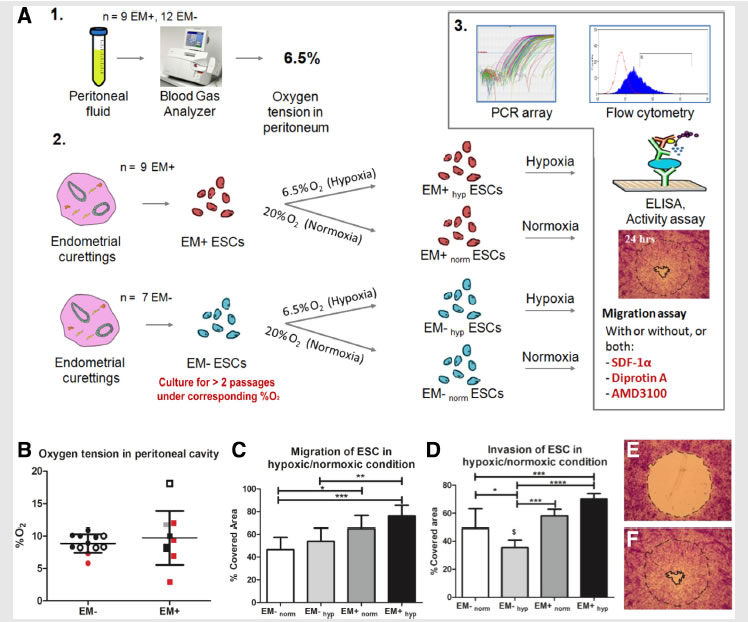
A collaborative study by researchers from BioSyM, MIT, KK Women's and Children Hospital, Singapore and Duke-NUS Graduate Medical School, Singapore on "Endometriosis" has uncovered that the loss of migration inhibitor under hypoxic conditions leads to enhanced endometrial stromal cell motility. The details have now been published in the journal "Fertility and Sterility"
Migration and invasion under physiological oxygen tension in endometriosis.
-
Prof. Lim Chwee Teck, Department of Biomedical Engineering, NUS and BioSyM Co-Investigator has won University Awards 2014 as an Outstanding Researcher.
-
Prof. Lim Chwee Teck, Department of Biomedical Engineering, NUS and BioSyM Co-Investigator adds another feather to his cap when he won the NUS Outstanding Innovator Award at the InnovFest 2014 organised by NUS Enterprise.
-
BioSyM researchers and collaborators from NUS, National Cancer Centre of Singapore (NCCS) and Clearbridge Biomedics have developed "An ultra-high throughput spiral microfluidic biochip for the enrichment of circulating tumor cells". The details are published in Analyst. This paper presents a simple yet reliable microfluidic device for the ultra-high throughput, label-free, size-based isolation of CTCs from clinically relevant blood volumes. The fast processing time of the technique (7.5 mL blood in less than 10 mins) and ability to collect more CTCs from larger blood volume lends itself to a broad range of potential genomic and transcriptomic applications.
-
BioSyM researchers and collaborators from Duke-NUS/KKH & NTU have demonstrated an Enhanced ex vivo expansion of adult mesenchymal stem cells by fetal mesenchymal stem cell Extra Cellular Matrix (ECM).
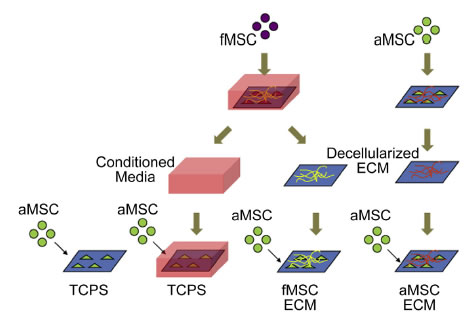
Schematic of decellularized ECM study. MSCs are plated on TCPS and cultured to generate extracellular matrices. The MSCs are decellularized to expose the layer of ECM for proliferation studies with aMSCs.
-
The Scientist Magazine highlights BioSyM's collaborative research on isolation and characterization of rare tumor cells circulating in the blood.
SPIRAL CHIP: A microfluidic biochip called ClearCell FX can isolate viable circulating tumor cells (CTCs). The blood sample and a saline buffer are pumped into separate inlets. As cells move through the channel, inertial and centrifugal forces ultimately cause the smaller red and white blood cells to flow along the channel’s outer wall and the larger CTCs to flow along the inner wall. The channel splits into two, where most blood cells flow toward a waste chamberand the CTCs flow toward a collection container. © CLEARBRIDGE BIOMEDICS PTE LTD
-
BioSyM researchers presented 3 papers on advanced bio-imaging technologies at the SPIE BiOS| SPIE Photonics West Conference (1-6 Feb 2014, San Francicso).
-
Vijay Raj Singh (SMART), Dipanjan Bhattacharya (SMART/NUS), Paul Matsudaria (NUS), George Barbastathis (MIT/SMART), Peter T. C. So (MIT/SMART), “Image reconstruction methodologies for structured light based laser sheet microscope for thick tissue imaging”, SPIE BiOS| SPIE Photonics West, 1-6 Feb 2014, San Francicso
-
Elijah Y. S. Yew (SMART), Peter T. C. So (MIT/SMART), "Improving the optical sectioning capability of temporally focused widefield two-photon microscopy" SPIE BiOS| SPIE Photonics West, 1-6 Feb 2014, San Francicso
-
Yubo Duan (NUS/SMART), Shakil Rehman (SMART), George Barbastathis (MIT/SMART) and Nanguang Chen (NUS), "Aperture design in focal modulation microscopy to improve
modulation depth", SPIE BiOS| SPIE Photonics West, 1-6 Feb 2014, San Francicso
-
Using a 3D microfluidic device and time-lapse confocal microscopy, BioSyM researchers show for the first time that Delta-like 4 (Dll4) containing exosomes causes tip cells to lose their filopodia and trigger capillary sprout retraction in collagen matrix. The details are published in Scientific Reports, 4, 4031 (2014). This study has demonstrated that exosomes are more than biomarkers and can be developed as a tool for drug delivery.

Dll4 exosomes reduced cell proliferation when applied in the gradient
-
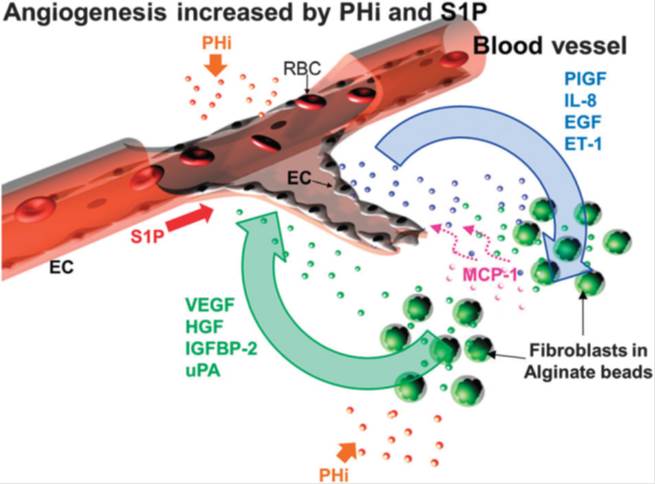
BioSyM researchers have utilized the proprietary 3D microfludic platform to understand angiogenic event that is driven by prolyl hydroxylase inhibitor (PHi) and sphingosine 1-phosphate (S1P) in the presence of fibroblast. The observations not only demonstrate the collaboration of EC and fibroblasts in inducing capillary sprouting but also suggest that the combination of CPX and S1P enhances angiogenesis and thus might be of therapeutic value for the pharmacological induction of tissue repair and regeneration. The details of this work are now published in the journal "Integrative Biology", 18, pp. 1474-84, 2013; http://www.ncbi.nlm.nih.gov/pubmed/24190477
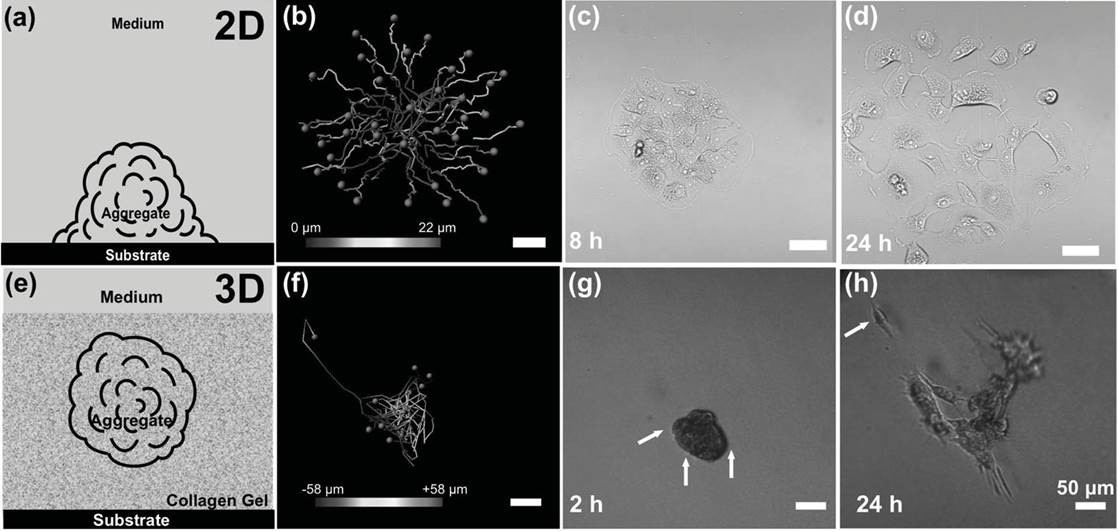
BioSyM researchers with their collaborators from A*STAR-IMCB and NUS/CSIS have developed a rapid, low-cost prototyping method to create microwells for the formation of 3D Multicellular Cancer Aggregates for Drug Screening applications. The details of this work are now published online in "Advanced Healthcare Materials", Aug, 2013.
Cancer Cell migration path in 2D and 3D conditions
-
BioSyM researchers along with collaborators from NUS and National Cancer Centre, Singapore have demonstrated the application of a novel microfluidic
platform for ultra-fast enrichment of putative CTCs using inertial microfluidics in spiral microchannels with a trapezoid cross-section ("Slanted spiral microfluidics for ultra-fast, label-free circulating tumor cells isolation", Lab on a chip, DOI: 10.1039/C3LC50617G, (Web), July 2013). The viability of the isolated CTCs was also retained after processing, which will allow potential culture and expansion studies. This work is highlighted by Chemistry World (http://www.rsc.org/chemistryworld/2013/07/circulating-tumour-cells-microfluidic-separation)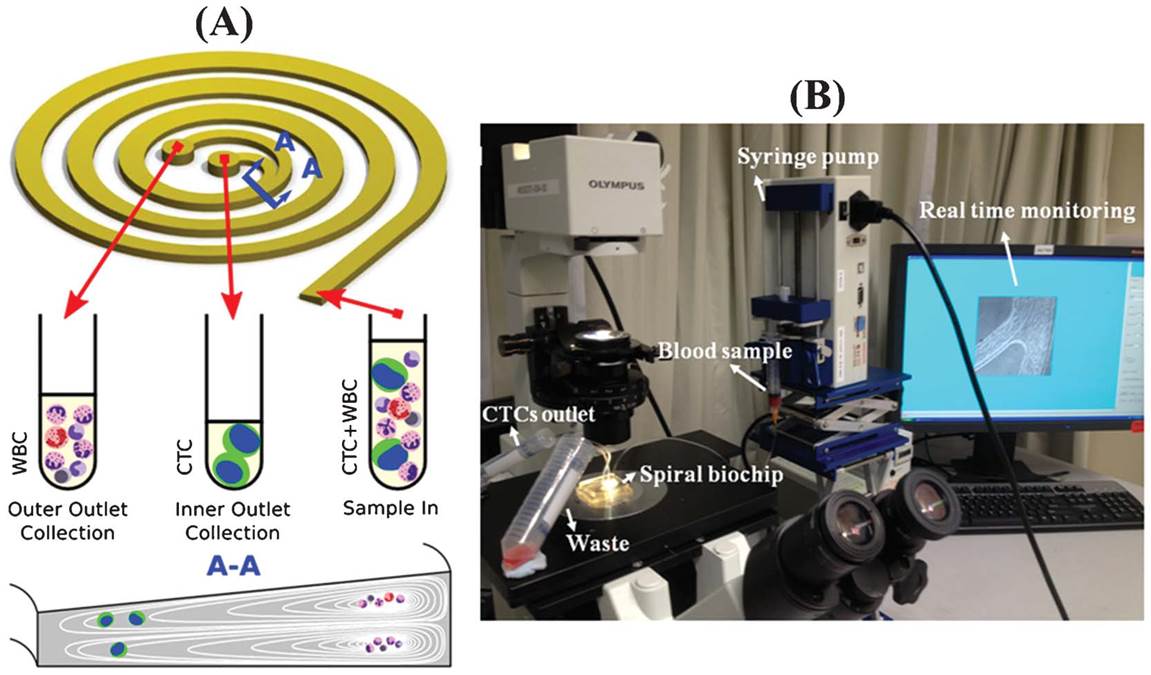
-
BioSyM's work on Two-Photon Microscopy and its applications to Bio-Imaging
-
BioSyM researchers in Singapore and MIT have have demonstrated the use of structured light illumination in Two Photon Microscopy to effectively reject background scattered emission photons and thereby improve image contrast when imaging in a turbid medium (“Improvement of axial resolution and contrast in temporally focused widefield two-photon microscopy with structured light illumination”, Biomed Opt Express, 4, 995—1005, 2013).
-
More of BioSyM's work on Two-Photon Microscopy published in "Temporally Focused Widefield Two-photon Microscopy: Paraxial to Vectorial," Optics Express, 21, 12951—12963, (2013)
-
-
BioSyM Start-Up, "AIM Biotech", founded by Prof. Roger D. Kamm, has been awarded Proof-Of-Value grant by SPRING Singapore on 1st April 2013. Established under SPRING's Technology Enterprise Commercialisation Scheme, the grant is awarded through a competitive process. AIM Biotech will be using the grant to develop a 3D cell culture microfluidic chip system, based on intellectual property from MIT and SMART, Singapore. The company targets to have devices ready for beta testing by the end of 2013
-
Confining DNA in microfluidic and nanofluidic devices has many practical applications, such as genome mapping. BioSyM researchers reveal the behaviour of DNA in confinement, specifically its dynamics, in the recently published paper in Physical Review Letters (Revisiting Blob Theory for DNA Diffusivity in Slitlike Confinement, Phys. Rev.Lett., 110, 168105 (2013))
-
BioSyM's paper on "Screening therapeutic EMT blocking agents in a three dimensional microenvironment" (http://www.ncbi.nlm.nih.gov/ pubmed/23172153), was amongst the top ten accessed articles from the online version of the journal "Integrative Biology". (http://blogs.rsc.org/ib/ category/top-ten/). This work is about cancer drug screening using 3D microfluidic device, one of the major research efforts @ BioSyM with Singapore collaborators at A*STAR-IMCB and CSIS-NUS
-
SMART BioSyM researchers add innovative microfluidic devices to their cell sorting technology portfolio. Details published in Scientific Reports (Nature Publishing Group)
-
Prof. Linda Griffith, BioSyM Principal Investigator have been named 2013 MacVicar Faculty Fellow for her outstanding undergraduate teaching, mentoring and educational innovation.
-
NUS and SMART Researchers revealed three distinct structural reorganizations of DNA by mechanical stretching (Details published in PNAS)
-
Singapore scientists at SMART Centre (BioSyM) developed novel biochip for identification and enrichment of circulating tumour cells (CTCs). Details published in Scientific Reports (Nature Publishing Group)
-
BioSyM has moved to their new lab in CREATE @ UTown, NUS
BioSyM IRG has completed its relocation to its new facility in CREATE (Sept/Oct-2012). The state of the art facility now includes wet labs in level 4 of the Enterprise Wing and bio-imaging labs in the basement.
-
Singapore Start-up Licenses SMART-BioSyM's Microfluidics Technology
-
Technology for thick and deep tissue imaging in real time: Embryo to Adult tissue development
SMART-BioSyM, Centre for Bio-Imaging Sciences (NUS) and Mechanobiology Institute study biological processes using BioSyM's Advanced Imaging Technologies and Bioinformatics tools
-
Prof. Steve Tannenbaum, SMART-BioSyM Principal Investigator, has been selected to receive the 2013 Division of Toxicology Founders Award from the American Chemical Society. This Award recognizes Steve's extraordinary career contributions to the field of toxicology broadly as well as to this ACS Division. It will be presented at the ACS National Meeting in September-2013, where Steve will give the Founders Award Lecture.
-
BioSyM has developed a miniaturized, portable Magnetic Resonance Relaxometry System for Point of Care Medical Diagnosis
-
Protein Impedes micro-circulation of Malaria infected blood Cells
-
Dr Zhang Baile, a former BioSyM post-doc, named one of the top 35 Innovators in the World by MIT's Technology Review
-
BioSyM Researcher Dr.Dipanjan Bhattacharya is delivering a talk on “Development of HiLo Based DSLM to understand tissue mechanics of embryogenesis” at NUS/TLL/NIBB Joint Practical Workshop on Genetics, Genomics & Imaging in Medaka and Zebrafish
-
BioSyM researcher Dr.Peng Weng Kung presented his new diagnostic technology at INNOVFEST 2012 under the theme "Medical Devices and Diagnostics".
-
Plenary Lecture @ ICRA 2012 (2012 IEEE International Conference on Robotics and Automation)
Prof.Harrya Asada, MIT Professor and SMART-BioSyM PI, delivered a Plenary Lecture @ ICRA 2012 under the Theme "Robots and Automation: Innovation for Tomorrow's Needs". He presented a talk on "Bio-Bots: Bio-Integrated Robotics Using Live Cells As Components". Click here for Video Recording of his presentation.
-
BioSyM graduate student Dr.Hou Hanwei's paper on "A microfluidics approach towards high-throughput pathogen removal from blood using margination" is highlighted on Gizmag magazine
-
SMART-BioSyM, MIT and NUS researchers demonstrate effects of macromolecular crowding on the cell and matrix materials
MIT news release
-
NUS and SMART-BioSyM Researchers publish in PNAS, novel findings on force-induced DNA structural transitions
-
Ms.Liu Yuchun, a collaborating graduate student of BioSyM from Mechanical Engineering Dept & Yong Loo Lin School of Medicine, NUS has won "Bone-Tec - 2011" Award for the Best Young Scientist. This is following her presentation of a research paper at "bone-tec-2011", The International Bone-Tissue-Engineering Congress, which provides a platform for some of the most outstanding young scientists in bone tissue engineering.
-
BioSyM Principal Investigator Prof.Steven Tannenbaum elected as 2011 Fellow of the American Chemical Society
-
SMART BioSyM researchers present papers @ BMES-2011
-
SMART BioSyM researchers present papers @MicroTAS-2011
-
BioSyM Principal Investigator Prof.Linda Griffith elected to National Academy of Engineering (NAE)
-
Cell Cycle Synchronization of Stem Cells Using Inertial Microfluidics
-
Invisibility Cloak Developed by Researchers at SMART Centre Voted Among Top 10 Breakthroughs of 2010 by Physics World
-
Making everyday objects invisible using natural crystals
-
Roger Kamm elected to the Institute of Medicine
-
High-throughput three-dimensional lithographic microfabrication
-
Dielectric metamaterial magnifier for far-field imaging
-
Microfluidics based cell sorting
-
An advanced microfluidic platform to study cellular responses in 3D microenvironments
-
NSF funds center to investigate the creation of biological machines
-
BioSyM PIs / Collaborators awarded SMART Innovation Centre Grants
-
SMART-Tohoku GCOE joint Workshop on Micro & Nano Bioengineering


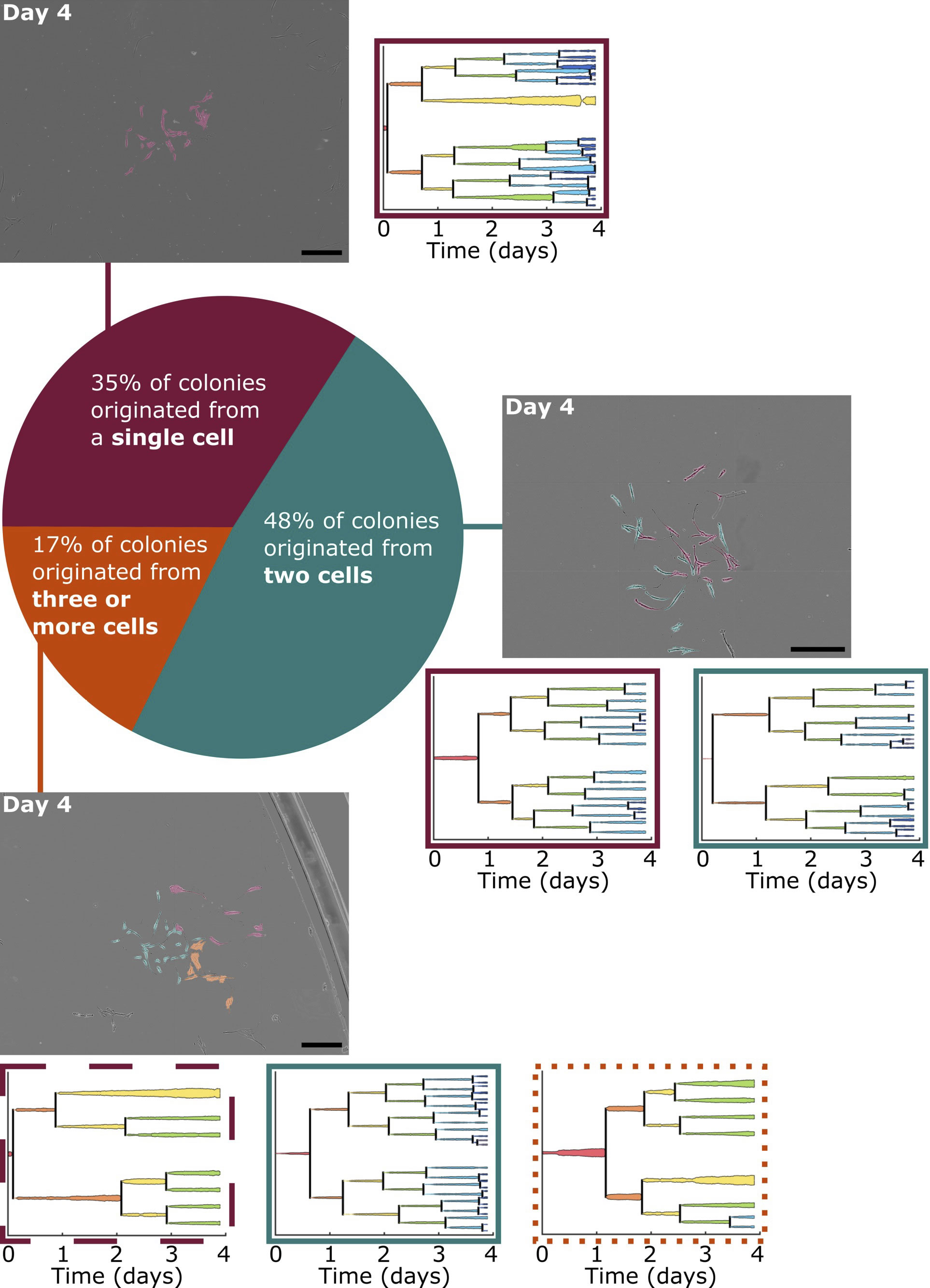
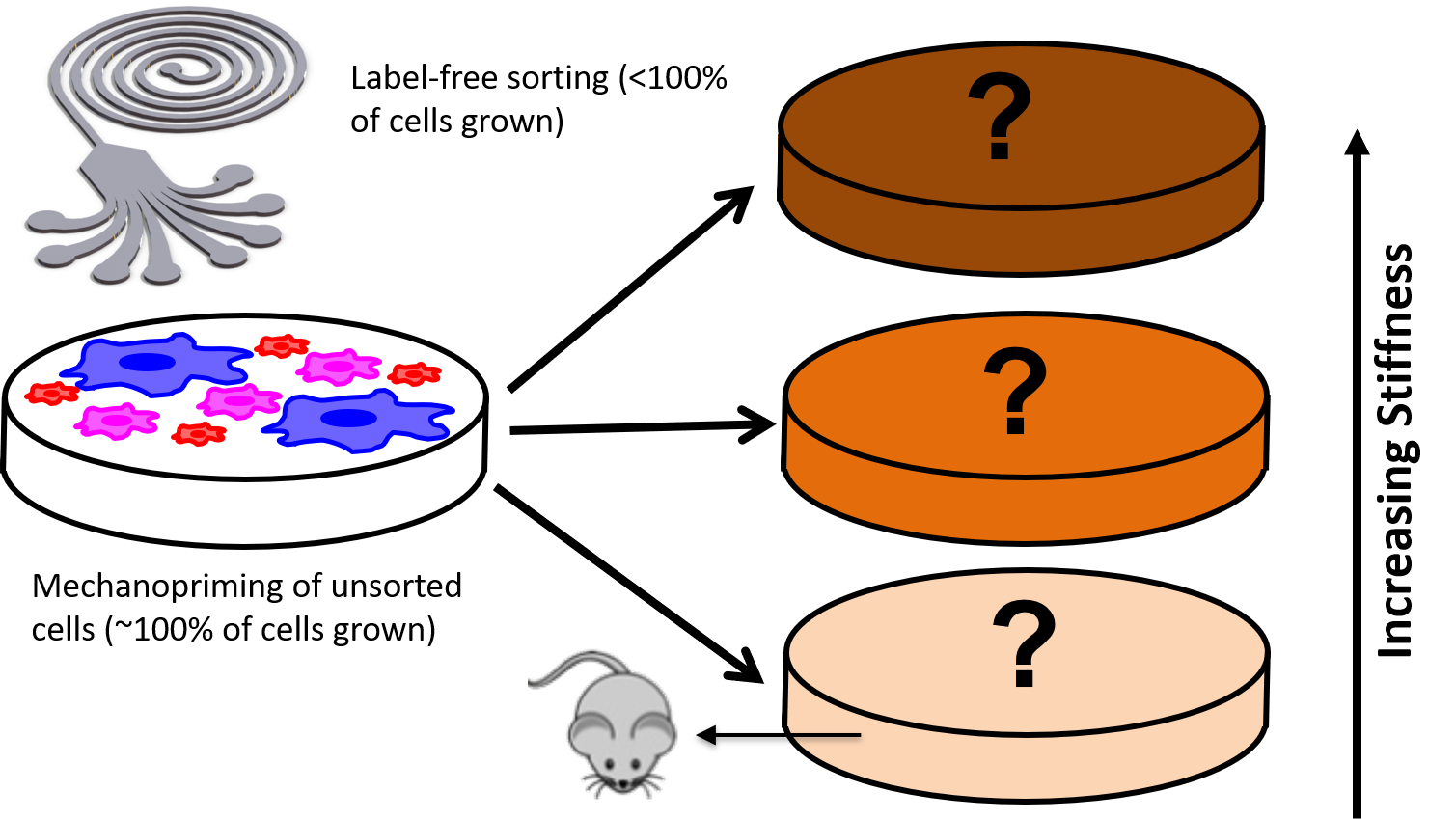
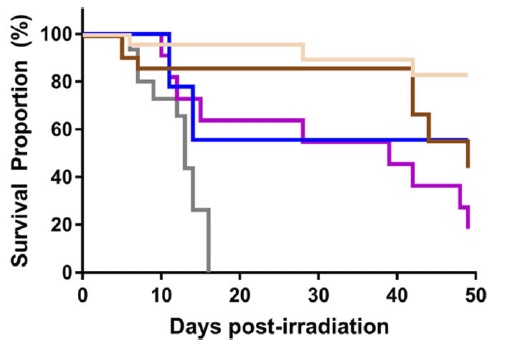 In-Vivo hematopoietic recovery
In-Vivo hematopoietic recovery